Biomed Industries Announces Phase 3 Trials of NA-931 Targeting Durable Weight Loss & Lean-Mass Preservation at JPM Week
Biomed Industries, Inc. Announces Two NA-931 Phase 3 Trials, Targeting Durable Weight Loss & Lean-Mass Preservation at JP Morgan Week 2026
Advancing NA-931, the first oral quadruple receptor agonist—into two Phase 3, as monotherapy and combination therapy, reflects our strategy to deliver durable weight-loss with lean-mass preservation.”
SAN JOSE, CA, UNITED STATES, January 19, 2026 /EINPresswire.com/ -- — Biomed Industries, Inc., a clinical-stage biopharmaceutical company developing next-generation therapies for obesity and metabolic disorders, today announced continued advancement of NA-931, a proprietary oral investigational therapy designed to deliver clinically meaningful weight reduction while supporting lean-mass preservation.— Dr. Lloyd L. Tran, CEO of Biomed
NA-931 is the first- in-class class oral quadruple receptor agonist designed to target IGF-1, GLP-1, GIP, and glucagon receptors. In the Company’s 13-week Phase 2 multiple ascending dose (MAD) study, NA-931 demonstrated dose-dependent reductions in mean body weight from baseline, including up to 13.8% at 150 mg once daily (11.9% relative to placebo). NA-931 was generally well tolerated, with an adverse event profile that Biomed believes was more favorable than published studies of semaglutide and tirzepatide, and no muscle loss was observed during the Phase 2 study.
“Obesity therapeutics have reached a new era of efficacy, but adoption at global scale still faces major barriers,” said Dr. Lloyd L. Tran, Chief Executive Officer of Biomed Industries. “NA-931 is being developed as a next-generation oral therapy intended to eliminate injections entirely, improve long-term persistence, and support healthier body composition outcomes—including lean-mass preservation.”
BIOMED ANNOUNCES PLANS FOR TWO GLOBAL PHASE 3 CLINICAL PROGRAMS
During 2026 J.P. Morgan Healthcare Conference Week, Biomed announced plans to initiate two global Phase 3 clinical programs evaluating NA-931 (120 mg orally once daily) as:
1) monotherapy, and
2) combination therapy with established GLP-1–based treatments
Biomed believes NA-931’s multi-pathway design may support more durable outcomes by simultaneously modulating appetite regulation, insulin sensitivity, energy expenditure, and IGF-1–mediated lean-mass preservation biology.
PLANNED PHASE 3 PROGRAM OVERVIEW
1. BIOCOMBO-1: NA-931 Alone and with Oral Semaglutide
BIOCOMBO-1 is a planned 68-week, randomized, double-blind, placebo-controlled, global Phase 3 study evaluating:
• NA-931 120 mg once daily (monotherapy)
• NA-931 120 mg once daily + oral semaglutide 25 mg once daily
Approximately 366 non-diabetic adults with overweight or obesity will be enrolled.
2. BIOCOMBO-2: NA-931 Alone and with Injectable Tirzepatide
BIOCOMBO-2 is a planned 68-week, randomized, double-blind, placebo-controlled, global Phase 3 study evaluating:
• NA-931 120 mg once daily (monotherapy)
• NA-931 120 mg once daily + injectable tirzepatide 7.5 mg once weekly
Approximately 360 non-diabetic adults with overweight or obesity will be enrolled, with aligned endpoints to support cross-study comparisons of weight loss magnitude, durability, and functional outcomes.
“Advancing NA-931 into two Phase 3 programs—monotherapy and combination—reflects our strategy to pursue durable weight loss outcomes while addressing one of the most important emerging concerns in obesity medicine: preservation of lean mass,” added Dr. Tran.
STRATEGIC MARKET POSITIONING: “ZERO INJECTIONS + LEAN-MASS PRESERVATION”
Biomed expects NA-931 to be positioned as a leading next-generation oral obesity therapy in a market still dominated by injectable method of administration. Adoption of injectables continues to face major limitations—particularly injection hesitation, long-term discontinuation, and practical barriers to scale globally.
Biomed believes NA-931 can help define the next era of obesity medicine by delivering two category-defining advantages: zero injections and a deliberate focus on lean-mass preservation, an emerging priority as the field shifts from “weight loss at any cost” to health span-driven, sustainable outcomes.
Skeletal muscle is a major component of body weight and is central to metabolic health. However, weight loss achieved with GLP-1–based therapies may also be accompanied by clinically significant lean-mass loss —raising concerns for long-term strength, physical function, and metabolic resilience. Biomed believes that the next generation of obesity therapies must deliver not only weight reduction, but higher-quality weight loss, where durable outcomes require protecting lean mass.
“Removing injections entirely is a game changer,” said Dr. Lloyd L. Tran, Chief Executive Officer of Biomed Industries. “We believe muscle preservation will define the next era of obesity medicine—because sustainable weight loss must protect strength, mobility, and long-term health.”
THE EMERGING ORAL ERA IN OBESITY CARE AND THE IMPORTANCE OF LONG-TERM PATENT PROTECTION
Patent protections for leading GLP-1 therapies are expected to begin expiring as early as 2026 in the U.S. and other markets—creating an upcoming significant “patent cliff” for the category. Biomed has secured worldwide patent protection covering NA-931, including composition-of-matter and methods-of-use claims, as well as intellectual property supporting combination therapy with established GLP-1–based treatments such as semaglutide and tirzepatide. Biomed believes this comprehensive IP strategy provides strong protection for NA-931 and its use in combination regimens through 2044.
Biomed’s long-term vision is to develop and commercialize NA-931 both as a stand-alone oral therapy and as a foundational oral component of next-generation combination regimens alongside other GLP-1 medicines in the market.
ABOUT BIOMED INDUSTRIES, INC.
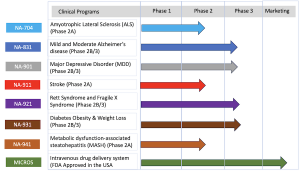
Biomed Industries, Inc. is a clinical-stage biopharmaceutical company focused on developing and commercializing transformative therapies for chronic and complex diseases. The company’s investigational pipeline targets a wide range of unmet medical needs, including:
- Alzheimer’s disease
- Major depressive disorder (MDD)
- Obesity and diabetes
- Metabolic dysfunction-associated steatohepatitis (MASH)
- Stroke and alcohol use disorder
- Rare diseases, including Rett Syndrome and Fragile X
For more information, please visit the website:
https://www.biomedind.com
FORWARD LOOKING STATEMENTS
This press release contains forward-looking statements regarding planned clinical trials, development timelines, and potential therapeutic benefits. Actual results may differ materially due to risks and uncertainties. Biomed undertakes no obligation to update forward-looking statements except as required by law.
Michael Willis
Biomed Industries, Inc.
+ +1 800-824-5135
email us here
Visit us on social media:
LinkedIn
X
Biomed introduction video
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.